Jiashun Chen | Baoju Kang | Yurong Zhao | Kang Yao | Chenxing Fu
引自Journal of Animal Physiology and Animal Nutrition学术期刊
摘要
本研究旨在调查日粮添加Sangrovit®仹犇威(SAG);0.15%的血根碱样品,一种从Macleaya cordata博落回提取获得的苯丙菲啶生物碱)对早期断奶仔猪的生长性能,肠道形态,肠道菌群及其代谢物的影响。共使用20头健康断奶仔猪(Duroc×[大白×长品种]),在21日龄断奶,平均体重(BW)为6.52±0.23公斤,被随机分配接受玉米 - 豆粕基础日粮(对照处理)或添加500 mg / kg Sangrovit 仹犇威(SAG)(博落回提取物)的基础日粮。共有20头健康断奶仔猪(Duroc×[大白×长品种]),在21日龄断奶,平均体重(BW)为6.52±0.23公斤,被随机分配接受玉米 - 豆粕基础日粮(对照处理)或添加500 mg / kg Sangrovit 仹犇威(SAG)(博落回提取物)的基础日粮。在为期21天的试验中,我们收集和分析了视网膜组织和腔内消化物的形态和肠道菌群,以及测量短链脂肪酸(SCFA)和氨的浓度。与对照处理组相比,添加Sangrovit 仹犇威(SAG)改善了平均日增重(p=0.011)和平均日采食量(p=0.037)。饲喂Sangrovit 仹犇威(SAG)日粮仔猪空肠隐窝深度(p = 0.011)的平均值较低,回肠绒毛高度(p = 0.015)以及空肠绒毛高度与窝窝深度之比(p < 0.01)和回肠(p = 0.027)高于接受对照处理日粮的动物。Sangrovit 仹犇威(SAG)的加入增加了回肠(p = 0.033)和盲肠(p < 0.01)中乳酸杆菌的含量,并倾向于增加盲肠中双歧杆菌的含量(p = 0.058),同时减少了回肠中大肠埃希菌(p = 0.046)和沙门氏菌属(p = 0.035)以及盲肠中沙门氏菌属(p = 0.029)的含量。与对照处理组相比,Sangrovit 仹犇威(SAG)膳食添加剂可提高(p < 0.05)乙酸盐、丙酸盐、丁酸盐和总SCFAs的浓度,并且还倾向于增加回肠和盲肠内容物中的戊酸盐水平(p = 0.055和p = 0.052)。在重新海绵状Sangrovit 仹犇威(SAG)的盲肠(p = 0.037)和回肠(p = 0.046)消化物中氨的浓度也下降。这些结果表明,饲喂添加Sangrovit 仹犇威(SAG)的早断奶仔猪可以潜在地改善其生长性能和肠道形态,并且可以以有益的方式改变肠腔环境。
关键词:生长性能、肠道形态、微生物群落、血根碱、断奶仔猪
介绍
仔猪早期断奶的做法现在在生猪生产中被广泛接受。然而,断奶压力会损害肠道结构和功能,损害肠道形态、微生物群以及免疫和消化系统(Pluske,汉普森和威廉姆斯,1997 年;尹等,2008)。肠道微生物群支持“肠道健康”,例如,哺乳动物消化道被密集动态和复杂的微生物群落所定植(Bäumler&Sperandio,2016),其产生的代谢物,如短链脂肪酸(SCFAs),乳酸和琥珀酸,可以作为肠上皮细胞的能量物质(Darcy-Vrillon等人,1993;Metzlerzebeli, Zijlstra, Mosenthin, & Gänzle, 2011;Piva, Casadei, & Biagi, 2002)。微生物种群还可以帮助确定动物饲料利用的效率(Fouhse,Zijlstra,&Willing,2016)。因此,肠道菌群在新陈代谢,免疫力发展和对病原体的抵抗力中起着至关重要的作用(Hooper&MacPherson,2010;李和长谷,2014)。抗生素已被广泛用于改善断奶仔猪的健康和生长性能(Frydendahl,2002;普罗斯克,2013 年)。然而,抗生素的滥用导致牲畜和人类的细菌耐药性,以及动物产品中的抗生素残留,这可能会给人类健康带来问题(Kemper,2008年;梦露和波尔克,2000;Schwarz, Kehrenberg, & Walsh, 2001)。Sangrovit® (Sangrovit 仹犇威(SAG)) 是一种天然博落回植物衍生的 (Macleaya cordata) 饲料添加剂,苯丙菲啶生物碱(QBA) 化合物,其中最丰富的是血根碱,一种具有抗菌剂的生物碱(Kosina 等人,2010 年;Newton, Lau, Gurcha, Besra, & Wright, 2001), 抗炎 (Niu, Fan, Li, Wei, & Huang, 2012;田中等人,1993;Yiu & Wei, 1993)和免疫调节活性(Chaturvedi等人,1997;Zhang, Ling, Chi, & Wang, 2013)。Sangrovit 仹犇威(SAG)膳食添加剂对猪有益(Jeroch等人,2009;Kantas, Papatsiros, Tassis, Athanasiou, & Tzika, 2015), 肉鸡 (Vieira, Berres, et al., 2008;Vieira, Oyarzabal, et al., 2008) 和 fish (Zhang et al., 2013)。断奶后仔猪日粮每公斤日粮添加120毫克M. cordata提取物可以证明生长性能和营养消化率(Goodarzi Boroojeni,Männer和Zentek,2018)。这种添加剂还可以影响胃肠道功能,包括胃肠道下部的肠道结构、运动和发酵过程(GIT)(Jankowski 等人,2009 年;科西纳等人,2004年)。在Juskiewicz等人(2011)对肉鸡进行的试验中,饲喂Sangrovit 仹犇威(SAG)对盲肠微生物群落活性有显着影响,并增加了盲肠消化物中总SCFA的浓度。
最近的一项研究表明,在肉鸡的日粮中添加20或50 ppm的Sangrovit 仹犇威(SAG)可以改善肉鸡的生长性能,增加相对空肠或回肠长度并改变肠道微生物群(Lee,Kim,&Oh,2015)。然而,以前的研究利用菌落计数方法来分析肠道菌群不能详细揭示微生物多样性。因此,近几十年来,聚合酶链反应(PCR)已成为快速,灵敏和特异性检测细菌生物的重要技术(Zhang等人,2013)。由于关于Sangrovit 仹犇威(SAG)如何影响肠道健康的信息很少,包括断奶仔猪的肠道形态、微生物群落和代谢特征,我们设计了这里介绍的实验,以研究Sangrovit 仹犇威(SAG)的日粮添加是否改变肠道形态、微生物群和相关代谢物,使用早期断奶仔猪作为我们的动物模型系统。
材料与方法
所有程序均由中国科学院亚热带农业研究所动物护理委员会批准。Sangrovit®仹犇威是德国仹犇泰公司的一种博落回提取物商业产品,由0.15%的血根碱作为活性成分的苯丙菲啶生物碱。
2.1 |实验设计,动物,日粮和饲养
在一项为期21天的实验中,共有20头健康断奶仔猪(杜鲁克×[大白×长种]),在21日龄时断奶,平均体重(BW)为6.52±0.23公斤,在随机完全区组设计中被分配到两个与性别和初始体重相关的日粮组。每组有10个重复(5个猪舍和5个后备母猪),每个重复1头猪。日粮处理包括玉米 - 豆粕基础日粮(对照处理)或添加500 mg / kg Sangrovit 仹犇威(SAG)的基础日粮。
两种日粮的配方均达到或超过国家研究委员会(2012)的营养需求(表1)。将个体动物饲养在温控育苗室(25-27°C)的不锈钢代谢笼(长150厘米×宽105厘米×高120厘米),相对湿度控制在60%-70%。提供自由清洁饮用水和指定的自由日粮采食。
表1. 实验日粮的成分和化学成分(以饲料为基础)
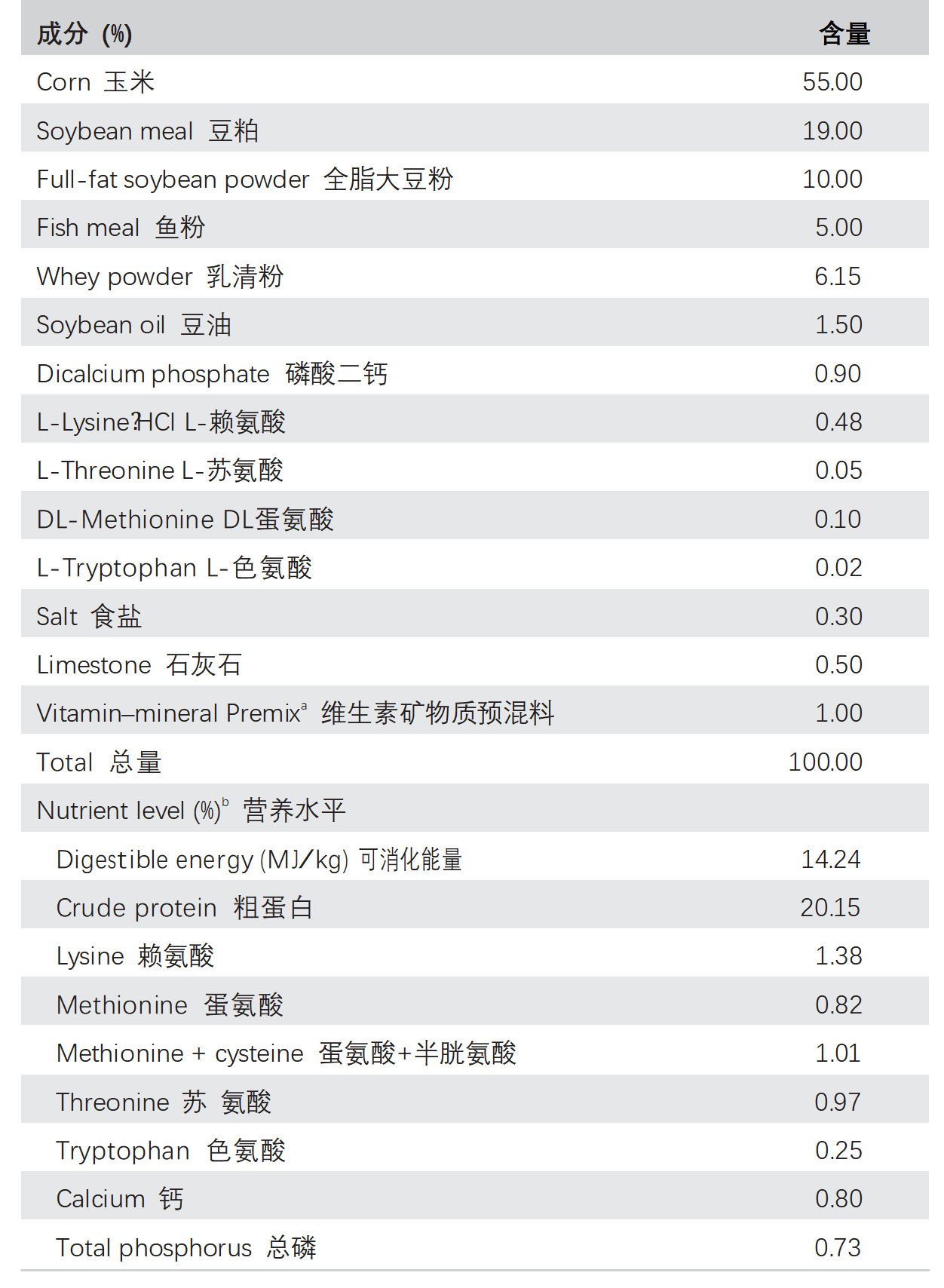
a维生素和矿物质预混料,每公斤饲料提供以下成分:维生素A,12,000 IU;维生素D,2,500 IU;维生素E,30 IU;维生素B12,12微克;维生素K,3毫克;D-泛酸,15毫克;烟酸,40毫克;氯化胆碱,400毫克;锰, 40 毫克;锌,100毫克;铁,90毫克;铜,8.8毫克;I,0.35毫克;硒,0.3 mg.b粗蛋白,钙和总磷分析值,其他值根据国家研究委员会(2012)计算值。
2.2 |样品采集和制备
在第21天测量个体体重和每个笼的饲料消耗量,以计算平均日增重(ADG),平均日增重(ADG),平均日采食量(ADFI)和增重与饲料比(G:F)。 当喂养试验结束时,所有动物都通过腹膜内注射戊巴比妥钠进行人道死亡,如Yao等人(2011)所述。之后,从每只动物身上取出整个肠道。收集十二指肠中部、空肠中部和回肠中部的节段进行形态学检查。同时,在测定SCFAs和氨的浓度以及肠道细菌的组成之前,收集盲肠和回肠的腔内消化物并将其储存在-80°C。
2.3 |通过实时荧光定量 PCR 进行细菌定量
根据先前描述的方案(Kraler,Ghanbari,Domig,Schedle和Kneifel,2016),使用QIAamp DNA粪便迷你试剂盒(德国Qiagen)从每个肠道样本的内容物(0.2g)中提取总细菌DNA。这些提取物储存在-80°C。 然后在Nanodrop 2000分光光度计(Thermo Scientific,Courtaboeuf,法国)上定量,然后将结果调整为10ng / μl的浓度。
如前所述,基于16S rRNA的方法用于评估总细菌,双歧杆菌,厚壁菌,大肠杆菌,乳酸杆菌和沙门氏菌属的丰富性(Fleury等人,2016;Kong等人,2014)。所有PCR引物均列于表2中。在含有1μl稀释DNA样品的混合物(最终体积,10μl)中进行重复样品分析,每个引物 0.2 μM 和 0.25 μM TaqMan® 探针,使用 1 × SYBR® 预混料 Ex Taq™ II 试剂盒(TaKaRa Bio Inc.,日本滋贺)。用于测定大肠杆菌的qPCR方案包括每个引物的0.3μM和0.1μM的探针,而厚壁菌的这些反应使用每个引物的浓度为0.4μM。扩增程序需要95°C持续30秒,然后是40个周期,95°C持续5秒,60°C持续30秒,然后是SYBR Green测试的最终熔化曲线。琼脂糖凝胶上的扩增的熔解曲线分析和尺寸测定验证了目标片段已被扩增。标准曲线由Qi等人(2011)描述生成。对于每个样品和每个细菌组,结果以每克肠道物质的16S rRNA基因的log10拷贝表达(Metzlerzebeli等人,2015)。
表2. 用于组特异性定量PCR的引物和探针序列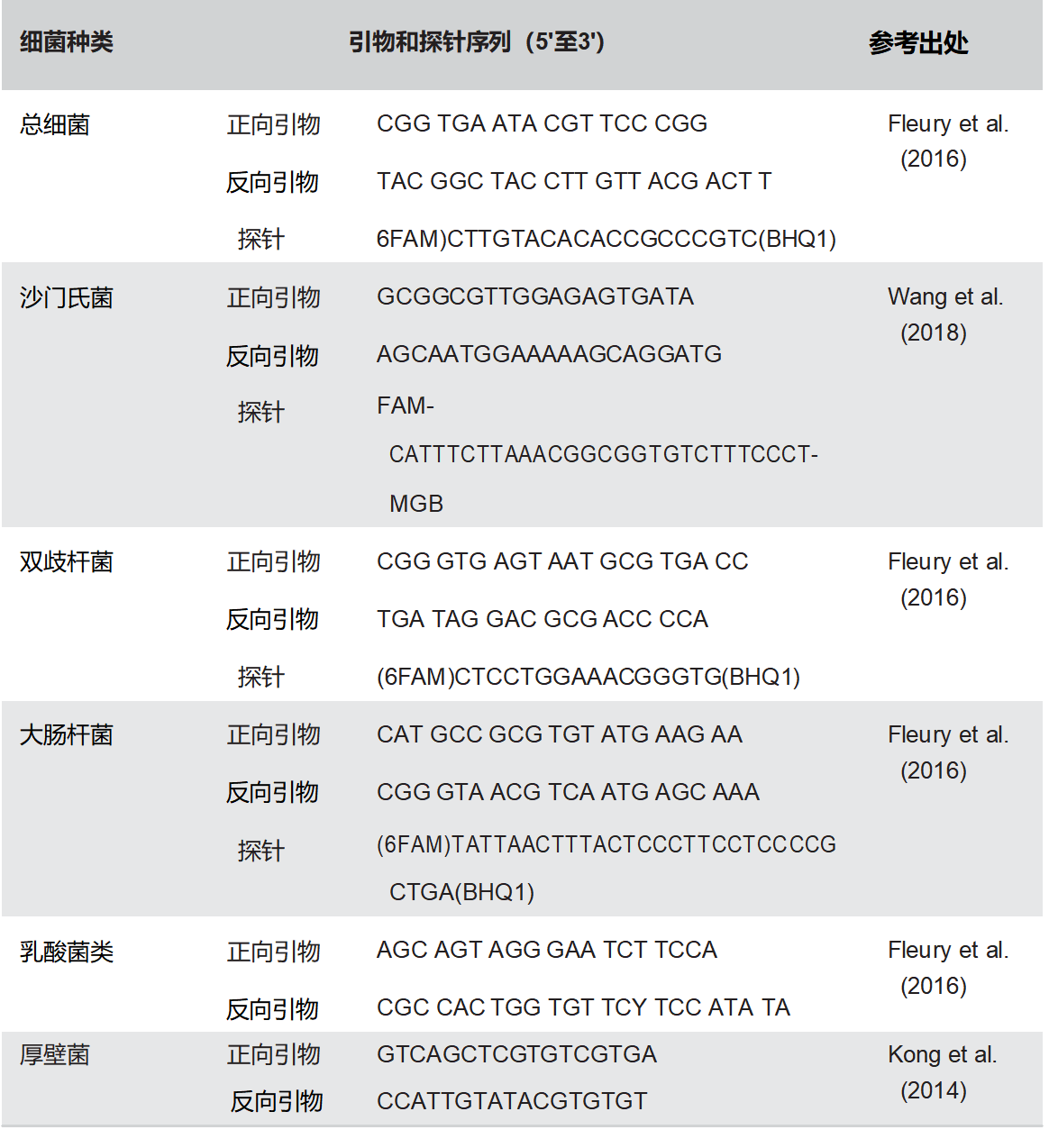
2.4 |细菌代谢物和水平分析肠道消化液中的氨
将回肠和盲肠样品的消化物匀浆并在1,000g和4°C下离心10分钟。然后,制备上清液和25%偏磷酸溶液(4ml:1ml)的混合物用于测定SCFAs(即乙酸,丙酸,丁酸,戊酸,异戊酸和异丁酸)。然后将上清液通过0.45μm聚砜过滤器,并在Agilent 6890气相色谱法(安捷伦科技公司,美国加利福尼亚州帕洛阿尔托)上进行分析,如前所述(Kong等人,2009)。氨浓度是根据Williams等人(2015)描述的方法计算的。
2.5 |形态学测量
将组织学样品快速固定在中性缓冲福尔马林中。将小肠切片切除、脱水并嵌入石蜡中,然后切割四个横向切片,放置在载玻片上并用苏木精和伊红染色。使用配备计算机辅助形态测量系统的尼康ECLIPSE 80i光学显微镜测量每段10个定向良好的绒毛的绒毛高度和隐窝深度(尼康公司,日本东京)(Chen等人,2016)。
2.6 |统计分析
使用t检验和SPSS软件进行统计分析(v.20.0;SPSS公司,美国伊利诺伊州芝加哥)。结果以均值和汇总标准误差的形式呈现。在p < 0.05时,处理之间的差异具有统计学意义,趋势根据0.05 < p < 0.10定义。
结果
3.1 |生长性能
在为期21天的研究中没有出现健康问题,也没有观察到拒绝喂养的情况。生长性能的参数如表3所示。在整个测试期间,饲喂添加500 mg / kg Sangrovit 仹犇威(SAG)的日粮的仔猪表现出更高的日增重ADG(p = 0.011)和平均日采食量ADFI(p = 0.037),并且与仅使用基础日粮喂养的动物相比,其增重与饲料比值(p = 0.058)趋于增加。
表3.日粮Sangrovit 仹犇威(SAG)添加剂对早断奶仔猪生长性能的影响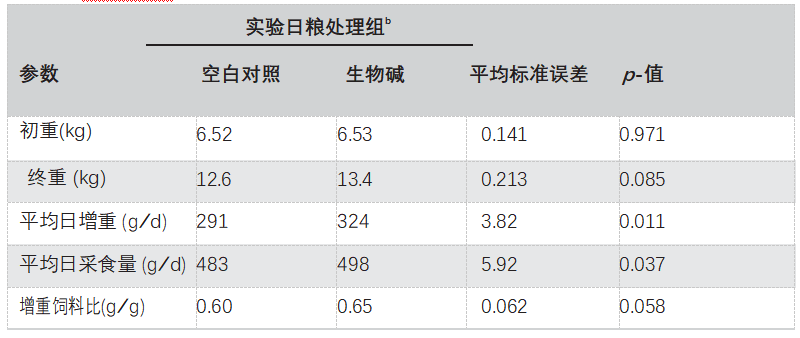
数据是每组十个重复(五个猪栏和五个后备母猪)和每个重复一个猪。SEM,平均值的合并标准误差。
日增重ADG:平均每日收益;平均日采食量ADFI:平均每日采食量;增重与饲料比:增重饲料比。
a猪(42日龄)从第0天到第21天(6-13公斤体重)饲喂实验性处理。Sangrovit 仹犇威(SAG),基础日粮,每公斤日粮添加500 毫克Sangrovit®。
3.2 |肠道形态学
表4列出了小肠形态的数据。与饲喂对照日粮的仔猪相比,接受Sangrovit 仹犇威(SAG)日粮的仔猪具有更高的回肠绒毛(p = 0.015)和更短的空肠隐窝(p = 0.011)。Sangrovit 仹犇威(SAG)仔猪空肠和回肠绒毛高度与窝窝深度的ra-tios大于对照处理组(p < 0.05)。此外,与对照处理动物的参数相比,膳食添加Sangrovit 仹犇威(SAG)导致绒毛高度降低(p = 0.046),并倾向于降低十二指肠绒毛高度与隐窝深度的比率(p = 0.077)。
表4. 日粮添加Sangrovit 仹犇威(SAG)对早断奶仔猪肠道形态的影响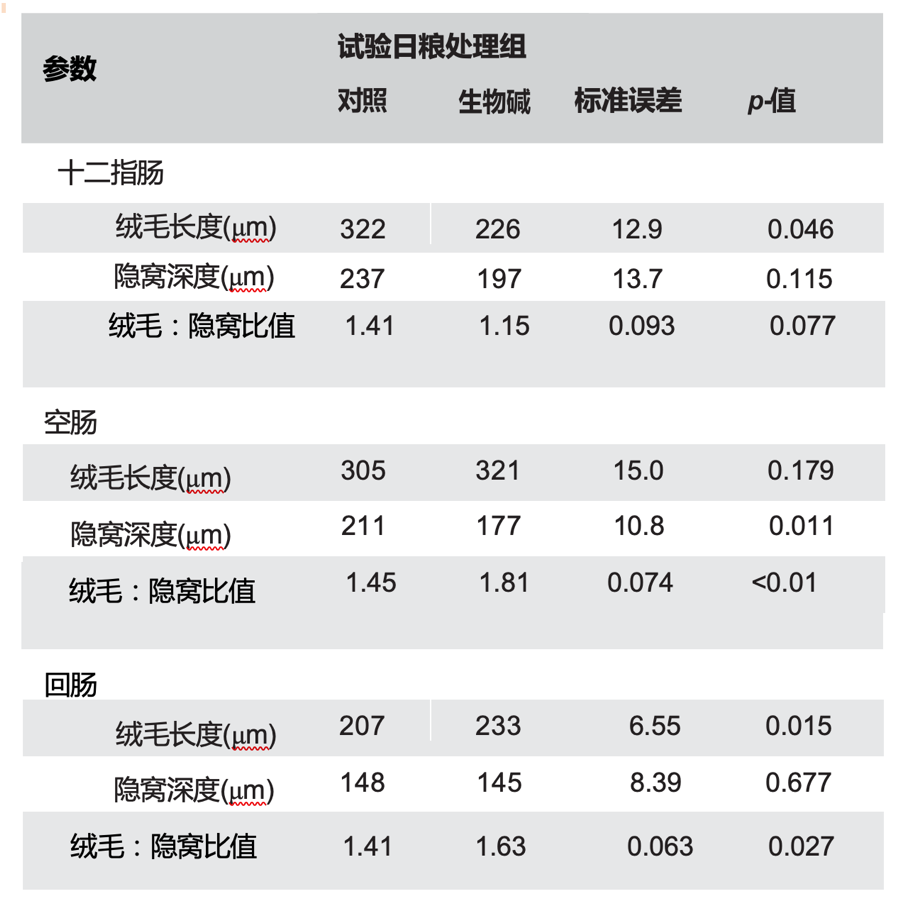
数据是每组十个重复(五个猪栏和五个后备母猪)和每个重复一个猪。SEM:平均值的合并标准误差。a对照处理:无添加剂;Sangrovit 仹犇威(SAG),基础日粮,每公斤日粮添加500 毫克Sangrovit®
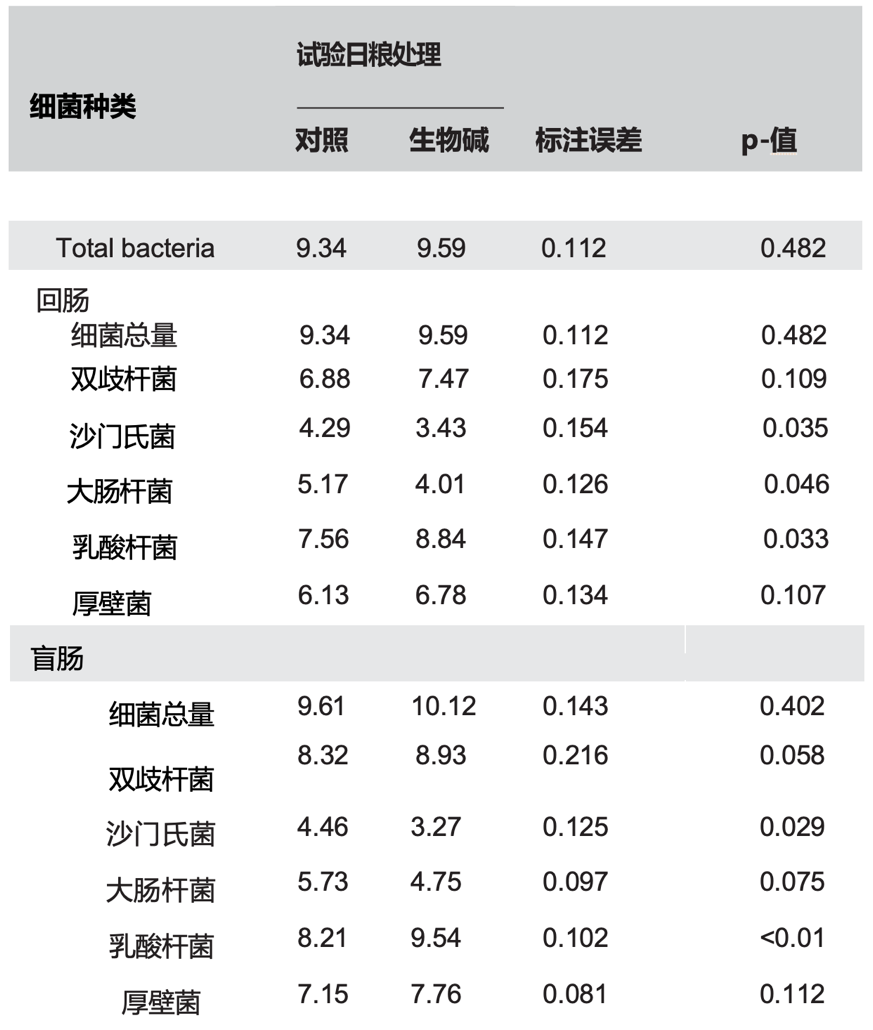
3.3 |肠道微生物群的定量
关于肠道菌群的详细信息如表5所示。与对照组相比,Sangrovit 仹犇威(SAG)的添加显着增加了回肠和盲肠中乳杆菌的含量(p<0.05),并倾向于增加盲肠中双歧杆菌的含量(p=0.058)。添加sangrovit p="">0.100)。与对照处理组的样品相比,膳食添加该化合物增加了乙酸盐,丙酸盐,丁酸盐和总SCFAs的浓度(p<0.05),并倾向于增加回肠和盲肠内容物中戊酸盐的水平(p = 0.055和p = 0.052),但降低了回肠和盲肠中的氨浓度(p <0.05)。
表5. 日粮添加Sangrovit 仹犇威(SAG)对早断奶仔猪肠道盲肠和回肠微生物种群的影响
讨论
研究显示,博落回提取物,如QBA异喹啉类生物碱化合物中的血根碱,被添加至猪,牛和家禽日粮可以促进生长,因为它们减少了由于脱羧和pH变化引起的氨基酸降解,同时增强了饲料消耗和消化(Drsata,Ulrichová和Walterová,1996;扬科夫斯基等人,2009 年;伦菲尔德等人,1981;Tschirner, Susenbeth, & Wolffram, 2003)。这些反应可能解释了为什么在我们的研究中接受这种添加剂的仔猪表现出更好的生长性能,即更高的日增重ADG和平均日采食量ADFI,比只喂食基础日粮的动物。且获得与Kantas等人(2015)的报告一致的结果,添加500 mg / kg Sangrovit 仹犇威(SAG)可改善断奶仔猪的日增重ADG和平均日采食量ADFI,并降低饲料转化率(FCR)。断奶后仔猪日粮每公斤日粮添加120毫克Cordata分枝杆菌提取物也改善了体重增加(BWG)和料比FCR(Goodarzi Boroojeni等人,2018)。然而,为生长中的猪(20-50公斤的体重)提供Sangrovit 仹犇威(SAG)(500 毫克/千克)对其生长性能或采食量没有影响(Blank,Müller-Siegwardt,&Wolffram,2010)。Vieira等人(2008a)发现,饲料添加剂Sangrovit 仹犇威(SAG)(125-500 ppm)可改善肉鸡雏鸡21天时的BW,35天的FCR和7日龄时的采食量。在另一项关于Cobb×Cobb雄性雏鸡喂养Sangrovit 仹犇威(SAG)的研究中(1-21天和22-42天分别为500 和250 ppm),动物在21天时表现出更好的BW值和更好的累积FCR(Vieira等人,2008b)。Sangrovit 仹犇威(SAG)对性能的这些对比影响可能与Sangrovit 仹犇威(SAG)的供应量,实验期的长度或测试的物种有关(Juskiewicz等人,2011;科西纳等人,2004年;罗林,梅里菲尔德和戴维斯,2009;薛等人,2017)。
肠道形态的变化是试图了解决定营养吸收能力变化的机制时应考虑的变量之一,受日粮调整的影响(Giannenas等人,2016)。由于肠绒毛是营养吸收的主要部位,绒毛高度的增加意味着更大的吸收面积(Cao等人,2013)。隐窝是产生新上皮细胞的绒毛工厂。更深的隐窝表明绒毛上皮更新更快速,以响应正常的脱屑或致病性炎症(Kamboh 等人,2015 年)。我们发现饲喂Sangrovit 仹犇威(SAG)日粮(500 mg / kg)的仔猪十二指肠的绒毛高度比对照处理处理的母猪短。
同样,Jankowski等人(2009年)报告说,用20毫克/千克血根碱制剂(M. cordata生物碱提取物)的日粮添加降低了肉鸡雏鸡十二指肠的绒毛高度。此外,我们还发现Sangrovit 仹犇威(SAG)添加剂与回肠绒毛较高,空肠和回肠绒毛高度与隐窝深度之比较大,空肠隐窝较浅有关,所有这些都表明这些仔猪具有增强的营养消化能力和吸收能力,有助于提高营养消化率和生长性能。Lee等人(2015)证明,饲喂添加20mg / kg Sangrovit 仹犇威(SAG)的肉鸡的相对空肠和回肠长度增加。在另一项蛋鸡实验中,与接受标准饲料混合物的母鸡相比,增加日粮中的血根碱水平导致空肠中的窝穴更浅,绒毛高度与窝穴深度比升高(Bavarsadi,Mahdavi,Ansari-Mahyari,&Jahanian,2016)。添加异喹啉生物碱也减少了受艾美氏菌攻击的肉鸡十二指肠、空肠和回肠病变的发生(Xue等人,2017 年)。
当猪被喂食添加2ppm(0.0002%)纯血根碱或100ppm(0.01%)血根碱制剂的日粮时,既没有观察到鼻窦营养不良,也没有观察到粘膜上皮炎症(Kosina等人,2004)。此外,血根碱可以增强生长仔猪的肠道屏障功能(Liu等人,2016)。含有异喹啉类生物碱QBAs(主要是血根碱)的商业产品表现出这些QBAs固有的抗菌特性(Lenfeld等人,1981)。在另一项研究中,Sangrovit 仹犇威(SAG)显着增加了肠道中的SCFAs(Juskiewicz等人,2011)。这些脂肪酸可以降低小肠的pH值并改善肠道的微生物种群。此外,由于微生物毒素对肠上皮的影响,病理学是通过微生物感染而发生的。已经发现,血根碱可以在第一次处理后的几个小时内将肠道壁从感染(例如梭状芽胞杆菌和大肠杆菌)中恢复的时间提速60%(Mellor,2001)。这表明血根碱抑制肠壁中有害细菌的作用,减少有毒化合物的产生和对肠上皮细胞的损害,从而保护肠粘膜(Yakhkeshi,Rahimi,&Naseri,2011)。我们在这里注意到的断奶仔猪肠道中的组织形态变化提供了关于采用Sangrovit 仹犇威(SAG)作为现在用于猪的抗生素生长促进剂的替代品的潜力的新信息。
以前的研究表明,药用植物提取物具有抗菌特性,有助于调节有机功能,肠道pH值和蠕动(Kommera,Mateo,Neher和Kim,2006;Kong等人,2007)。血根碱的抗菌作用已从许多体外和体内实验中报道(Herrera-Mata,Rosas-Romero和Oscar Crescente,2002;马哈德里亚,彭德兰和斯托亚,2003年;沃克,1990年;Zhao, Yu, Zhou, Meng, & Wu, 2005)。在本实验中,我们发现饲料中Sangrovit 仹犇威(SAG)的存在增加了从回肠和盲肠中采样的内容物中乳酸杆菌和双歧杆菌的水平,但降低了大肠杆菌和沙门氏菌属的水平。据我们所知,这是第一份关于Sangrovit 仹犇威(SAG)对断奶仔猪肠道微生物种群影响的报告。我们的发现与Yakhkeshi等人(2011)的研究结果一致,他们表明膳食Sangrovit 仹犇威(SAG)增加了肉鸡回肠和盲肠含量中乳酸菌的数量。Pickler等人(2013)报告说,与接受白开水的对照雏鸡测量的水平相比,通过饮用水施用血根碱可减少肠炎沙门氏菌在肠炎沙门氏菌中肠道沙门氏菌计数。Bavarsadi等人(2016)指出,膳食添加血根碱可抑制蛋鸡中大肠杆菌和沙门氏菌的回肠计数,而Miao等人(2011)表明血根碱是大肠杆菌,嗜水气单胞菌和金黄色沙门氏菌感染的有效抑制剂。
生物碱制备Sangrovit对肠道菌群的上述调节作用也得到了我们为总SCFA谱图计算的变化的证实。日粮添加Sangrovit 仹犇威(SAG)增加了回肠和盲肠样品中乙酸盐、丙酸盐、丁酸盐、戊酸盐和总SCFA的浓度。同样,Juskiewicz等人(2011)表明,添加Sangrovit 仹犇威(SAG)可提高SCFA浓度,从而降低肉鸡盲肠中的pH值。肠道pH值的下降有助于最大限度地减少肠道病原体的浓度,缓解幼畜断奶后腹泻(Kantas等人,2015年)。Juskiewicz等人(2011)还报告说,在饲料日粮中添加Sangrovit 仹犇威(SAG)改善了肉鸡盲肠消化物中α-葡萄糖苷酶,α-半乳糖苷酶和β-半乳糖苷酶的活性。这些酶促进乳糖,棉子糖家族低聚糖和抗性淀粉的发酵,导致SCFAs和乳酸的产生,作为这些动物的能量来源(Holt,Teresi和Côté,2008;Wierzbicka-Wos, Bartasun, Cieslinski, & Kur, 2013)。
在本研究中,用Sangrovit 仹犇威(SAG)添加我们的仔猪饲料可降低回肠和盲肠中的氨水平。这与Jankowski等人(2009年)的发现相似,他们显示接受Sangrovit 仹犇威(SAG)添加剂的肉鸡盲肠消化液中的氨浓度降低。当蛋白质和尿素被大肠中的细菌降解时氨是一种天然产物(Walsh等人,2007)。血根碱的分子结构与芳香族氨基酸的分子结构相似,例如色氨酸和苯丙氨酸。血根碱可以抑制芳香族氨基酸脱羧酶(AAD)的活性,以减少色氨酸和苯丙氨酸作为肠道中吲哚和粪臭素等有毒生物胺的损失(Mellor,2001)。这种酶抑制的结果是动物可以使用更多的芳香族氨基酸,从而促进更好的蛋白质平衡,从而从胴体中提高瘦肉的百分比。因此,我们仔猪肠道消化物中弹药浓度的降低表明添加Sangrovit 仹犇威(SAG)降低了盲肠生态系统中的蛋白水解活性和/或对GIT下部的饲料蛋白利用率产生积极影响。
结论
本研究结果表明,添加Sangrovit 仹犇威(SAG)(500 mg / kg)的基础日粮对早期断奶仔猪的生长性能,视网膜形态和微生物群落种群具有有益影响。特别是,我们证市,与标准饲料相比,接受这种添加剂的动物在其肠腔内容物中表现出乳酸杆菌和SCFA的含量增加,并抑制大肠杆菌,沙门氏菌属和氨的水平降低。
参考文献
Bäumler, A. J., & Sperandio, V. (2016). Interactions between the microbi- ota and pathogenic bacteria in the gut. Nature, 535, 85–93. https:// doi.org/10.1038/nature18849
Bavarsadi, M., Mahdavi, A. H., Ansari-Mahyari, S., & Jahanian, E. (2016). Effects of different levels of sanguinarine on antioxidant indices, im- munological responses, ileal microbial counts and jejunal morphol- ogy of laying hens fed diets with different levels of crude protein. Journal of Animal Physiology and Animal Nutrition, 101, 936–948.
Blank, R., Müller-Siegwardt, B., & Wolffram, S. (2010). Sanguinarine does not influence availability or metabolism of tryptophan in pigs. Livestock Science, 134, 24–26. https://doi.org/10.1016/j. livsci.2010.06.086
Cao, G. T., Zeng, X. F., Chen, A. G., Zhou, L., Zhang, L., Xiao, Y. P., & Yang, C. M. (2013). Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Science, 92, 2949–2955. https://doi.org/10.3382/ ps.2013-03366
Chaturvedi, M. M., Kumar, A., Darnay, B. G., Chainy, G. B., Agarwal, S., & Aggarwal, B. B. (1997). Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-κB activation, IκBα phosphorylation, and deg- radation. Journal of Biological Chemistry, 272, 30129–30134. https:// doi.org/10.1074/jbc.272.48.30129
Chen, Y. P., Cheng, Y. F., Li, X. H., Zhang, H., Yang, W. L., Wen, C., & Zhou, Y. M. (2016). Dietary palygorskite supplementation improves immunity, oxidative status, intestinal integrity, and barrier function of broilers at early age. Animal Feed Science and Technology, 219, 200– 209. https://doi.org/10.1016/j.anifeedsci.2016.06.013
Darcy-Vrillon, B., Morel, M. T., Cherbuy, C., Bernard, F., Posho, L., Blachier, F., … Duee, P. H. (1993). Metabolic characteristics of pig colonocytes after adaptation to a high fiber diet. The Journal of Nutrition, 123, 234–243.
Drsata, J., Ulrichová, J., & Walterová, D. (1996). Sanguinarine and chelerythrine as inhibitors of aromatic amino acid decarbox- ylase. Journal of Enzyme Inhibition, 10, 231–237. https://doi. org/10.3109/14756369609036530
Fleury, M. A., Jouy, E., Eono, F., Cariolet, R., Couet, W., Gobin, P., … Kempf, I. (2016). Impact of two different colistin dosing strategies on healthy piglet fecal microbiota. Research in Veterinary Science, 107, 152–160. https://doi.org/10.1016/j.rvsc.2016.06.003
Fouhse, J. M., Zijlstra, R. T., & Willing, B. P. (2016). The role of gut mi- crobiota in the health and disease of pigs. Journal of Animal Science, 6, 30–36.
Frydendahl, K. (2002). Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Veterinary Microbiology, 85, 169–182. https://doi.org/10.1016/ S0378-1135(01)00504-1
Giannenas, I., Doukas, D., Karamoutsios, A., Tzora, A., Bonos, E., Skoufos, I., … Florou-Paneri, P. (2016). Effects of Enterococcus fae- cium, mannan oligosaccharide, benzoic acid and their mixture on growth performance, intestinal microbiota, intestinal morphology and blood lymphocyte subpopulations of fattening pigs. Animal Feed Science and Technology, 220, 159–167. https://doi.org/10.1016/j. anifeedsci.2016.08.003
Goodarzi Boroojeni, F., Männer, K., & Zentek, J. (2018). The impacts of Macleaya cordata extract and naringin inclusion in post-weaning piglet diets on performance, nutrient digestibility and intestinal histomorphology. Archives of Animal Nutrition, 72, 178–189. https:// doi.org/10.1080/1745039x.2018.1459342
Han, K. N., Kwon, I. K., Lohakare, J. D., Heo, S., & Chae, B. J. (2007). Chito-oligosaccharides as an alternative to antimicrobials in im- proving performance, digestibility and microbial ecology of the gut in weanling pigs. Asian-Australasian Journal of Animal Sciences, 20, 556–562. https://doi.org/10.5713/ajas.2007.556
Herrera-Mata, H., Rosas-Romero, A., & Oscar Crescente, V. (2002). Biological activity of “Sanguinaria” (Justicia secunda) extracts. Pharmaceutical Biology, 40, 206–212. https://doi.org/10.1076/ phbi.40.3.206.5826
Holt, S. M., Teresi, J. M., & Côté, G. L. (2008). Influence of alternansucrase- derived oligosaccharides and other carbohydrates on α-galactosidase and α-glucosidase activity in Bifidobacterium adolescentis. Letters in Applied Microbiology, 46, 73–79.
Hooper, L. V., & MacPherson, A. J. (2010). Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Reviews Immunology, 10, 159–169. https://doi.org/10.1038/nri2710
Jankowski, J., Zduńczyk, Z., Ju?kiewicz, J., Koz?owski, K., Lecewicz, A., & Jeroch, H. (2009). Gastrointestinal tract and metabolic response of broilers to diets with the Macleaya cordata alkaloid extract. Archiv fur Geflugelkunde, 73, 95–101.
Jeroch, H., Kozlowski, K., Jeroch, J., Lipinski, K., Zduńczyk, Z., & Jankowski, J. (2009). Efficacy of the phytogenic (Papaveraceae) ad- ditive Sangrovit® in growing monogastric animals. Züchtungskunde, 81, 279–293.
Juskiewicz, J., Gruzauskas, R., Zduńczyk, Z., Semaskaite, A., Jankowski, J., Totilas, Z., … Svirmickas, G. (2011). Effects of dietary addition of Macleaya cordata alkaloid extract on growth performance, caecal indices and breast meat fatty acids profile in male broilers. Journal of Animal Physiology and Animal Nutrition, 95, 171–178. https://doi. org/10.1111/j.1439-0396.2010.01037.x
Kamboh, A. A., Arain, M. A., Mughal, M. J., Zaman, A., Arain, Z. M., & Soomro, A. H. (2015). Flavonoids: Health promoting phytochemicals for animal production – A review. Animal Health Products, 3, 6–13. https://doi.org/10.14737/journal.jahp
Kantas, D., Papatsiros, V. G., Tassis, P. D., Athanasiou, L. V., & Tzika, E. D. (2015). The effect of a natural feed additive (Macleaya cordata), con- taining sanguinarine, on the performance and health status of wean- ing pigs. Animal Science Journal, 86, 92–98. https://doi.org/10.1111/ asj.12240
Kemper, N. (2008). Veterinary antibiotics in the aquatic and terrestrial environment. Ecological Indicators, 8, 1–13. https://doi.org/10.1016/j. ecolind.2007.06.002
Kommera, S. K., Mateo, R. D., Neher, F. J., & Kim, S. W. (2006). Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Australasian Journal of Animal Sciences, 19, 1784–1789. https://doi.org/10.5713/ajas.2006.1784
Kong, X. F., Wu, G. Y., Liao, Y. P., Hou, Z. P., Liu, H. J., Yin, F. G., … Deng, D. (2007). Effects of Chinese herbal ultra-fine powder as a dietary addi- tive on growth performance, serum metabolites and intestinal health in early-weaned piglets. Livestock Science, 108, 272–275. https://doi. org/10.1016/j.livsci.2007.01.079
Kong, X. F., Zhang, Y. Z., Wu, X., Yin, Y. L., Tan, Z. L., Feng, Y., … Li, T. J. (2009). Fermentation characterization of Chinese yam polysaccha- ride and its effects on the gut microbiota of rats. International Journal of Microbiology, 2009, 598152.
Kong, X. F., Zhou, X. L., Lian, G. Q., Blachier, F., Liu, G., Tan, B. E., … Yin, Y. L. (2014). Dietary supplementation with chitooligosaccharides alters gut microbiota and modifies intestinal luminal metabolites in weaned Huanjiang mini-piglets. Livestock Science, 160, 97–101. https://doi. org/10.1016/j.livsci.2013.11.023
Kosina, P., Gregorova, J., Gruz, J., Vacek, J., Kolar, M., Vogel, M., … Ulrichova, J. (2010). Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia, 81, 1006–1012. https://doi. org/10.1016/j.fitote.2010.06.020
Kosina, P., Walterová, D., Ulrichová, J., Lichnovský, V., Stiborová, M., Rýdlová, H., … Simánek, V. (2004). Sanguinarine and chelerythrine: Assessment of safety on pigs in ninety days feeding experiment. Food and Chemical Toxicology, 42, 85–91. https://doi.org/10.1016/j. fct.2003.08.007
Kraler, M., Ghanbari, M., Domig, K. J., Schedle, K., & Kneifel, W. (2016). The intestinal microbiota of piglets fed with wheat bran variants as characterised by 16S rRNA next-generation amplicon sequencing. Archives of Animal Nutrition, 70, 173–189. https://doi.org/10.1080/1 745039X.2016.1160534
Lee, W. J., & Hase, K. (2014). Gut microbiota-generated metabolites in animal health and disease. Nature Chemical Biology, 10, 416–424. https://doi.org/10.1038/nchembio.1535
Lee, K. W., Kim, J. S., & Oh, S. T. (2015). Effects of dietary sangui- narine on growth performance, relative organ weight, cecal micro- flora, serum cholesterol level and meat quality in broiler chickens. Journal of Poultry Science, 52, 15–22. https://doi.org/10.2141/ jpsa.0140073
Lenfeld, J., Kroutil, M., Marsálek, E., Slavík, J., Preininger, V., & Simánek, V. (1981). Anti-inflammatory activity of quaternary benzophenan- thridine alkaloids from Chelidonium majus. Planta Medica, 43, 161– 165. https://doi.org/10.1055/s-2007-971493
Liu, G., Guan, G., Fang, J., Martínez, Y., Chen, S., Peng, B., … Al-Dhabi, N. A. (2016). Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. BioMed Research International, 2016, 1–7.
Mahadria, G. S., Pendland, A., & Stoia, L. (2003). In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canaden- sis and Hydrastis canadensis. Phytotherapy Research, 17, 217–221.
Mellor, S. (2001). Natural appetisers from plants. Feed Mix, 9, 29–31. Metzlerzebeli, B. U., Schmitzesser, S., Mann, E., Grüll, D., Molnar, T., &
Zebeli, Q. (2015). Adaptation of the cecal bacterial microbiome of growing pigs in response to resistant starch type 4. Applied and Environmental Microbiology, 81, 8489–8499. https://doi.org/10.1128/ AEM.02756-15
Metzlerzebeli, B. U., Zijlstra, R. T., Mosenthin, R., & Gänzle, M. G. (2011). Dietary calcium phosphate content and oat β-glucan influence gas- trointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiology Ecology, 75, 402– 413. https://doi.org/10.1111/j.1574-6941.2010.01017.x
Miao, F., Yang, X. J., Zhou, L., Hu, H. J., Zheng, F., Ding, X. D., … Sun, W. (2011). Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Natural Product Research, 25, 863– 875. https://doi.org/10.1080/14786419.2010.482055
Monroe, S., & Polk, R. (2000). Antimicrobial use and bacterial resistance. Current Opinion in Microbiology, 3, 496–501. https://doi.org/10.1016/ S1369-5274(00)00129-6
National Research Council (2012). Nutrient requirements of swine, 11th rev ed. Washington, DC: National Academy Press.
Newton, S. M., Lau, C., Gurcha, S. S., Besra, G. S., & Wright, C. W. (2001). The evaluation of forty-three plant species for in vitro antimycobac- terial activities; isolation of active constituents from Psoralea coryli- folia and Sanguinaria canadensis. Journal of Ethnopharmacology, 79, 57–67.
Niu, X., Fan, T., Li, W., Wei, X., & Huang, H. (2012). The anti- inflammatory effects of sanguinarine and its modulation of in- flammatory mediators from peritoneal macrophages. European Journal of Pharmacology, 689, 262–269. https://doi.org/10.1016/j. ejphar.2012.05.039
Pickler, L., Beirão, B. C. B., Hayashi, R. M., Durau, J. F., Lourenço, M. C., Caron, L. F., & Santin, E. (2013). Effect of sanguinarine in drinking water on Salmonella control and the expression of immune cells in peripheral blood and intestinal mucosa of broilers. Journal of Applied Poultry Research, 22, 430–438. https://doi.org/10.3382/ japr.2012-00649
Piva, A., Casadei, G., & Biagi, G. (2002). An organic acid blend can mod- ulate swine intestinal fermentation and reduce microbial proteol- ysis. Canadian Journal of Animal Science, 82, 527–532. https://doi. org/10.4141/A01-090
Pluske, J. R. (2013). Feed-and feed additives-related aspects of gut health and development in weanling pigs. Journal of Animal Science and Biotechnology, 4, 83–89.
Pluske, J. R., Hampson, D. J., & Williams, I. H. (1997). Factors influenc- ing the structure and function of the small intestine in the weaned pig: A review. Livestock Production Science, 51, 215–236. https://doi. org/10.1016/S0301-6226(97)00057-2
Qi, H., Xiang, Z., Han, G., Yu, B., Huang, Z., & Chen, D. (2011). Effects of different dietary protein sources on cecal microflora in rats. African Journal of Biotechnology, 10, 3704–3708.
Rawling, M. D., Merrifield, D. L., & Davies, S. J. (2009). Preliminary assessment of dietary supplementation of Sangrovit® on red ti- lapia (Oreochromis niloticus) growth performance and health. Aquaculture, 294, 118–122. https://doi.org/10.1016/j.aquaculture. 2009.05.005
Schwarz, S., Kehrenberg, C., & Walsh, T. R. (2001). Use of antimicro- bial agents in veterinary medicine and food animal production. International Journal of Antimicrobial Agents, 17, 431–437. https://doi. org/10.1016/S0924-8579(01)00297-7
Tanaka, T., Metori, K., Mineo, S., Hirotani, M., Furuya, T., & Kobayashi, S. (1993). Inhibitory effects of berberine-type al- kaloids on elastase. Planta Medica, 59, 200–202. https://doi. org/10.1055/s-2006-959651
Tschirner, K., Susenbeth, A., & Wolffram, S. (2003). Influence of Sangrovit supplementation on nitrogen balance and feed intake in growing pigs. In: 9th Symposium on Vitamins and Additives in Nutrition of Man and Animal, Jena/Thuringia, Germany. Friedrich Schiller Univ., Jena. P. 45.
Vieira, S. L., Berres, J., Reis, R. N., Oyarzabal, O. A., Coneglian, J. L. B., Freitas, D. M., … Torres, C. A. (2008). Studies with sangui- narine like alkaloids as feed additive in broiler diets. Brazilian Journal of Poultry Science, 10, 67–71. https://doi.org/10.1590/ S1516-635X2008000100010
Vieira, S. L., Oyarzabal, O. A., Freitas, D. M., Berres, J., Peña, J. E. M., Torres, C. A., & Coneglian, J. L. B. (2008). Performance of broilers fed diets supplemented with sanguinarine-like alkaloids and organic acids. Journal of Applied Poultry Research, 17, 128–133. https://doi. org/10.3382/japr.2007-00054
Walker, C. (1990). Effects of sanguinarine and Sanguinaria extract on the microbiota associated with the oral cavity. The Journal of the American Dental Association, 56, 13–30.
Walsh, M. C., Sholly, D. M., Hinson, R. B., Trapp, S. A., Sutton, A. L., Radcliffe, J. S., … Richert, B. T. (2007). Effects of acid LAC and Kem- Gest acid blends on growth performance and microbial shedding in weanling pigs. Journal of Animal Science, 85, 459–467. https://doi. org/10.2527/jas.2005-630
Wang, M., Yang, J. J., Gai, Z. T., Huo, S. G., Zhu, J. H., Li, J., … Zhang,L. (2018). Comparison between digital PCR and real-time PCR in detection of Salmonella typhimurium in milk. International Journal of Food Microbiology, 266, 251–256. https://doi.org/10.1016/j.ijfoodmicro.2017.12.011
Wierzbicka-Wos, A., Bartasun, P., Cieslinski, H., & Kur, J. (2013). Cloning and characterization of a novel cold-active glycoside hydrolase fam- ily 1 enzyme with β-glucosidase, β-fucosidase and β-galactosidase activities. BMC Biotechnology, 13, 1–12.
Williams, B. A., Zhang, D., Lisle, A. T., Mikkelsen, D., McSweeney, C. S., Kang, S., … Gidley, M. J. (2015). Soluble arabinoxylan enhances large intestinal microbial health biomarkers in pigs fed a red meat- containing diet. Nutrition, 32, 491–497.
Xue, G. D., Wu, S. B., Choct, M., Pastor, A., Steiner, T., & Swick, R. A. (2017). Impact of a Macleaya cordata-derived alkaloid extract on ne- crotic enteritis in broilers. Poultry Science, 96, 3581–3585. https:// doi.org/10.3382/ps/pex164
Yakhkeshi, S., Rahimi, S., & Naseri, K. G. (2011). The effects of com- parison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, GIT microbial population, intestinal morphology and performance of broilers. Journal of Medicinal Plants Research, 10, 80–95.
Yao, K., Guan, S., Li, T., Huang, R., Wu, G., Ruan, Z., & Yin, Y. (2011). Dietary L-arginine supplementation enhances intestinal develop- ment and expression of vascular endothelial growth factor in wean- ling piglets. British Journal of Nutrition, 105, 703–709. https://doi. org/10.1017/S000711451000365X
Yin, Y. L., Tang, Z. R., Sun, Z. H., Liu, Z. Q., Li, T. J., Huang, R. L., … Chen, L. X. (2008). Effect of galacto-mannan-oligosaccharides or chitosan supplementation on cytoimmunity and humoral immunity in early- weaned piglets, Asian-Australas. Journal of Animal Science, 21, 723– 731. https://doi.org/10.5713/ajas.2008.70408
Yiu, C. K., & Wei, S. H. (1993). Clinical efficacy of dentifrices in the con- trol of calculus, plaque, and gingivitis. Quintessence International, 24, 181–188.
Zhang, C., Ling, F., Chi, C., & Wang, G. X. (2013). Effects of praziquantel and sanguinarine on expression of immune genes and susceptibility to Aeromonas hydrophila in goldfish (Carassius auratus) infected with Dactylogyrus intermedius. Fish and Shellfish Immunology, 35, 1301– 1308. https://doi.org/10.1016/j.fsi.2013.08.001
Zhao, X., Li, L., Luo, Q., Ye, M., Luo, G., & Kuang, Z. (2015). Effects of mul- berry (Morus alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livestock Science, 177, 88–94. https://doi.org/10.1016/j. livsci.2015.03.001
Zhao, D. L., Yu, J. P., Zhou, X. Q., Meng, X. B., & Wu, J. Z. (2005). Antibacterial effect of the sanguinarine hydrochloride and boccono-line from Macleaya cordata. Food Science, 26, 45–47.

-
[06-25]
-
[06-15]
-
[05-22]
-
[05-12]
-
[04-23]
-
[04-14]
-
[04-07]
-
[02-18]
-
[02-03]
-
[01-13]




